IOMEPROL:
| Active name: | IOMEPROL |
| Brand name: | IOMERVU |
| Innovator name | BRACCO DIAGNOSTICS INC |
| Approval Date: | NOV 27, 2024 |
| Therapeutic activity: | Tri-iodinated X-Ray contrasting agent. Iomeprol has been investigated for the diagnostic of Coronary Artery Disease. |
| Route of administration | Intra arterial/intravenous, Injection |
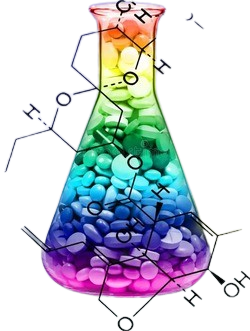
| Structure | |
| Chemical name | N,N’-bis(2,3-dihydroxypropyl)-5-[(hydroxyacetyl)- methylamino]-2,4,6-tri-iodo-1,3-benzenedicarboxamide. |
| CAS | 78649-41-9 |
| Dosage | Available in four iodine concentration. 510 mg (250 mg rebound iodine), 612 mg (300 mg rebound iodine); 714 mg (350 mg rebound iodine); 816 mg (400 mg rebound iodine); |
| Molecular weight& molecular formuale | 777.09 g/mol & C17H22I3N3O8 (Iodine content 49%) |
| Solubility | water |
| NCE | 11/27/2029 |
| Solubility data | Soluble in water. |
| Hygroscopicity | Not hygroscopic |
| Drugbank | DB11705 |
| Chemspider | 3600 |
| Pubchem CID | 3731 |
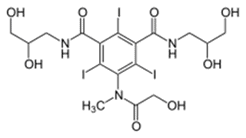
Synthesis of Iomeprol:
ROS-1: Ref: EP 0026281A1
Iomeprol started with commercially available 5-amino-2,4,6-triiodoisophthalic acid, which was couple with formamide followed by thionoyl chloride to get the corresponding acid chloride. Coupling of acid chloride with 3-amino-1,2-propylene glycol to achieve the Iomeprol.
1H-NMR (DMSO-D 6)δ(ppm):2.91(s, 4H),3.21-3.36(m, 4H), 3.41-3.65(m, 4H), 3.85(s, 2H), 3.98(m,2H), 4.1-4.5(m,5H), 8.2-8.4(d, 2H); 13C-NMR(D 2O)δ(ppm)33.2,44.7,60.7,64.6,71.2,90.1,99.8,99.9,145.5,150.5,150.6,171.3,171.4,173.9.
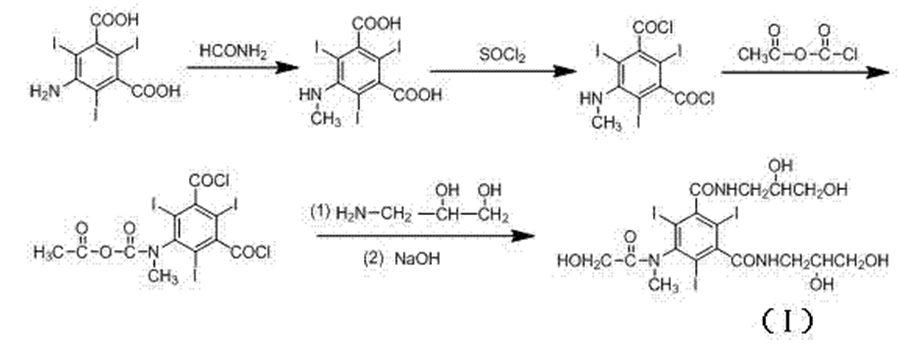
ROS-2: CN 102363600B
Iomeprol started with commercially available 5-amino-2,4,6-triiodoisophthalic acid, which was couple with thionoyl chloride followed bychloro acetyl chloride to get the corresponding acid chloride. Coupling of acid chloride with 3-amino-1,2-propylene glycol, followed by treated with the sodium acetate to achieve the Iomeprol.