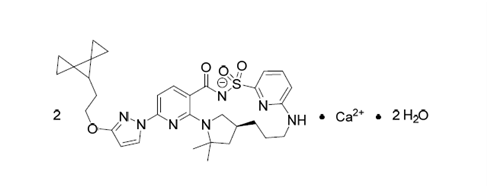
| Active name: | Vanzacaftor calcium dihydrate |
| Brand name: | ALYFTREK |
| Innovator name | VERTEX PHARMACEUTICALS INC |
| Approval Date: | Dec 20, 2024 |
| Therapeutic activity: | Binds to the different sites on the CFTR protein and have an additive effect in facilitating the cellular processing and trafficking of select mutant forms of CFTR (F508del-CFTR) to increase the amount of CFTR Protein delivered to the cell su |
| Route of adminstration | Tablet, oral |
| Structure | |
| Chemical name | calcium bis((14S)-8-[3-(2-{dispiro[2.0.2⁴.1³]heptan-7-yl}ethoxy)pyrazol-1- yl]-12,12-dimethyl-2,2,4-trioxo-2λ⁶-thia-3,9,11,18,23-pentaazatetracyclo[17.3.1.1¹¹, ¹⁴.0⁵ , ¹⁰]tetracosa-1(23),5,7,9,19,21- hexaen-3-ide) dihydrate |
| CAS | 2374124-49-7 |
| Dosage | 4 mg base eq (4.24 mg of vanzacaftor calcium dehydrate)& 10 mg base eq (10.6 mg of vanzacaftor calcium dehydrate) |
| Molecular weight& molecular formuale | 654.82 g/mol & C32H38N7O4S.Ca0.5.H2O |
| OB patents | US7495103B2; US7645789 B2; US7776905B2; US 8623905 |
| Polymorhic patent | US9487525 B2; |
| NCE | 12/20/2029 |
| Solubility data | Insoluble in water. |
| Hygroscopicity | Not hygroscopic |
| Drugbank | DB18373 |
| Chemspider | 115010436 |
Orange book Patent Data
| Patent No | Patent Expiration | Drug Substance | Drug Product | Patent Use Code | Delist Requested | Submission Date |
| 7495103 | 05/20/2027 | DS | DP | 01/17/2025 | ||
| 7645789 | 05/01/2027 | DS | DP | 01/17/2025 | ||
| 7776905 | 06/03/2027 | DS | DP | 01/17/2025 | ||
| 8324242 | 08/05/2027 | U-4090 | 01/17/2025 | |||
| 8354427 | 07/06/2026 | U-4091 | 01/17/2025 | |||
| 8410274 | 12/28/2026 | DP | 01/17/2025 | |||
| 8415387 | 11/12/2027 | U-4082 | 01/17/2025 | |||
| 8598181 | 05/01/2027 | U-4090 | 01/17/2025 | |||
| 8623905 | 05/01/2027 | DS | DP | 01/17/2025 | ||
| 8629162 | 06/24/2025 | U-4084 | 01/17/2025 | |||
| 8754224 | 12/28/2026 | DS | DP | 01/17/2025 | ||
| 8865902 | 05/17/2032 | DS | DP | 01/17/2025 | ||
| 9181192 | 05/17/2032 | DS | DP | U-4094 | 01/17/2025 | |
| 9512079 | 05/17/2032 | DS | DP | U-4098 | 01/17/2025 | |
| 9670163 | 12/28/2026 | DP | U-4080 | 01/17/2025 | ||
| 9931334 | 12/28/2026 | DP | U-4080 | 01/17/2025 | ||
| 9974781 | 04/09/2027 | DP | U-4082 | 01/17/2025 | ||
| 10022352 | 04/09/2027 | DP | U-4081 | 01/17/2025 | ||
| 10047053 | 05/17/2032 | DS | 01/17/2025 | |||
| 10081621 | 03/25/2031 | DP | U-4087 | 01/17/2025 | ||
| 10239867 | 04/09/2027 | DS | DP | U-4090 | 01/17/2025 | |
| 10646481 | 08/13/2029 | DP | 01/17/2025 | |||
| 11066417 | 02/14/2039 | DS | DP | 01/17/2025 | ||
| 11564916 | 08/13/2029 | U-4095 | 01/17/2025 | |||
| 11578062 | 03/25/2031 | DP | U-4096 | 01/17/2025 | ||
| 11639347 | 04/09/2027 | DS | DP | U-4090 | 01/17/2025 | |
| 11866450 | 02/14/2039 | U-4082 | 01/17/2025 | |||
| 11873300 | 08/13/2040 | DS |
Cystic fibrosis (CF) is a genetic condition caused by mutations in the CFTR gene, which controls ion and fluid transport across epithelial cells. Mutations cause consequences, the most severe of which is life-limiting lung illness.Traditional therapies aimed to manage symptoms, but improvements in understanding CF’s molecular foundation resulted in small-molecule CFTR modulators. Ivacaftor, a potentiator, is approved for gating mutations. Dual combinations, such as ivacaftor/lumacaftor and ivacaftor/tezacaftor, combined a potentiator and a class 1 corrector for F508del homozygous patients. Most CF patients now receive triple-combination CFTR modulators, such as ivacaftor/tezacaftor/elexacaftor with an additional class 2 corrector, which has transformed the disease’s outlook. Vanzacaftor/tezacaftor/deutivacaftor comes under class 2 corrector.