| Active name: | Pirtobrutinib |
| Brand name: | Jaypirca |
| Innovator name | LOXO ONCOLOGY ING |
| Approval Date: | Jan 27, 2023 |
| Therapeutic activity: | kinase inhibitor used to treat relapsed or refractory mantle cell lymphoma (MCL) after at least two lines of systemic therapy. |
| Route of adminstration | Tablet, oral |
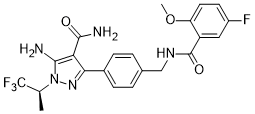
| Structure | |
| Chemical name | 5-amino-3-{4-[(5-fluoro-2-methoxybenzamido)methyl]phenyl}-1-[(2S)- 1,1,1-trifluoropropan-2-yl]-1H-pyrazole-4-carboxamide |
| CAS | 2101700-15-4 |
| Dosage: | 50 &100 mg |
| MDD | 200 mg |
| Molecular weight& molecular formuale | 479.44 & C22H21F4N5O3 |
| OB patents | US 10342780 B2; US10464905 B2; US10695323 B2 (12/16/2036) |
| Polymorhic patent | US 2021330643A1; WO2022240920A1; WO2020028258A1 |
| NCE | 01/27/2028; ODE-424 01/27/2028; ODE-451 12/01/2030 |
| Solubility data | Insoluble in water range pH 1 to 7 |
| Hygroscopicity | Not hygroscopic |
| Pubchem CID | 129269915 |
| Drugbank | DB17472 |
| Chemspider | 114875989 |
Patent Data
| Patent No | Patent Expiration | Drug Substance | Drug Product | Submission Date |
| 10342780 | 12/16/2036 | DS | DP | 02/10/2023 |
| 10464905 | 12/16/2036 | 02/10/2023 | ||
| 10695323 | 12/16/2036 | DS | DP | 02/10/2023 |
| 10918622 | 12/16/2036 | 02/10/2023 | ||
| 12109193 | 09/14/2041 | DP | 10/31/2024 |
Synthesis:
Scheme-1: Ref: US 10342780B2
The synthesis of Pirtobrutinib started with 5-Fluoro-2-methoxy benzoic acid, react with thionyl chloride followed by amidation with 4-(aminomethyl)benzoicacid to form intermediate3. Intermediate 3 treated with thionyl chloride followed by malononitrile to afford Intermediate II. Intermediate-II couple with compound 8 to give the Pirtobrutinib. This was disclosed in WO2022/056100.
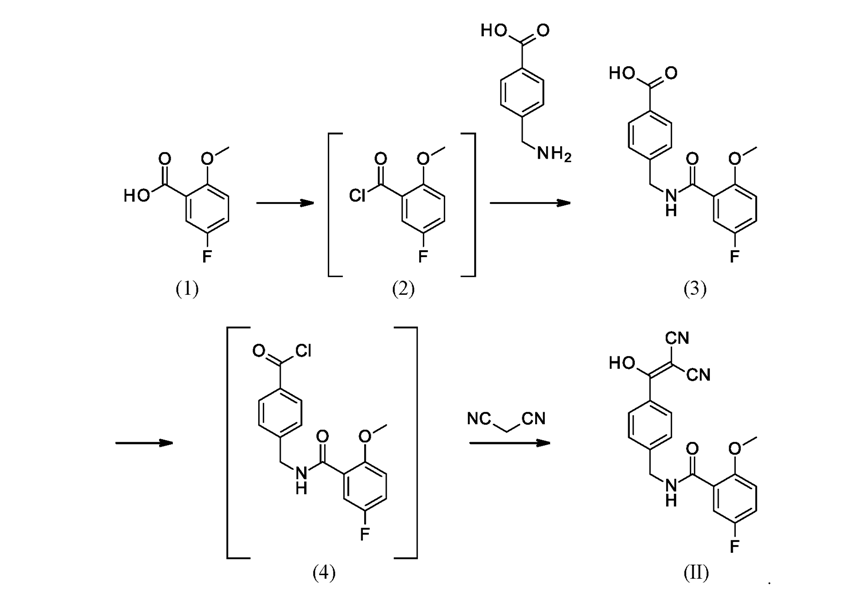
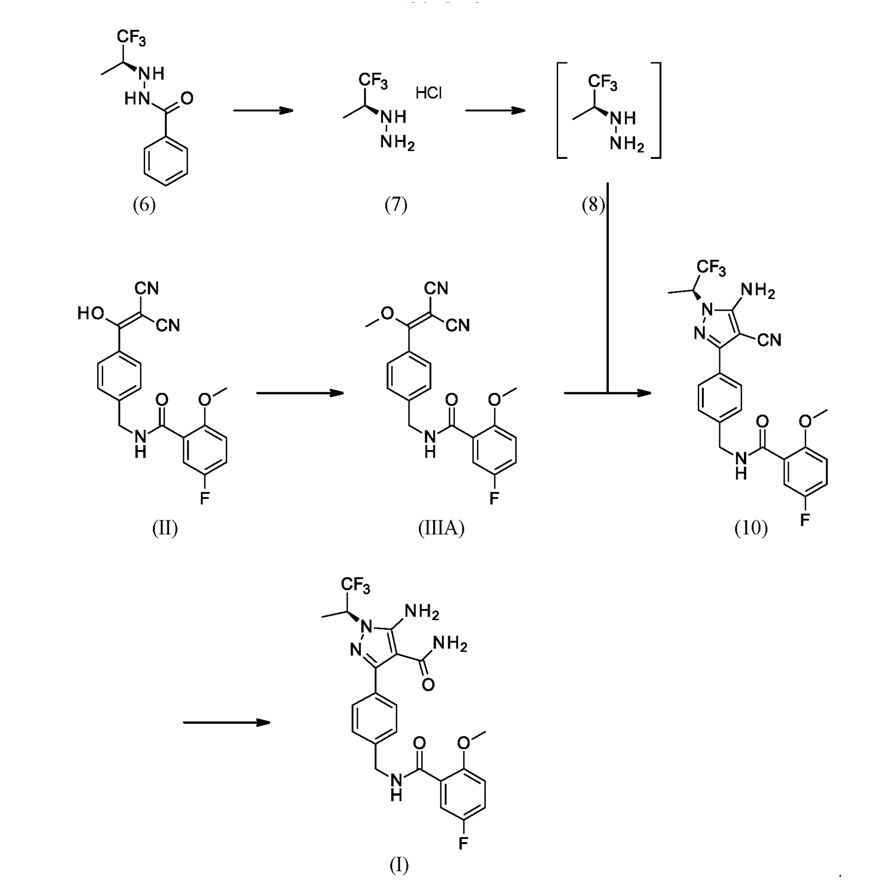

Scheme-2: Ref: WO2022/056100



Scheme-3: Ref: WO2022/056100


Scheme-4: Ref: WO2022/056100
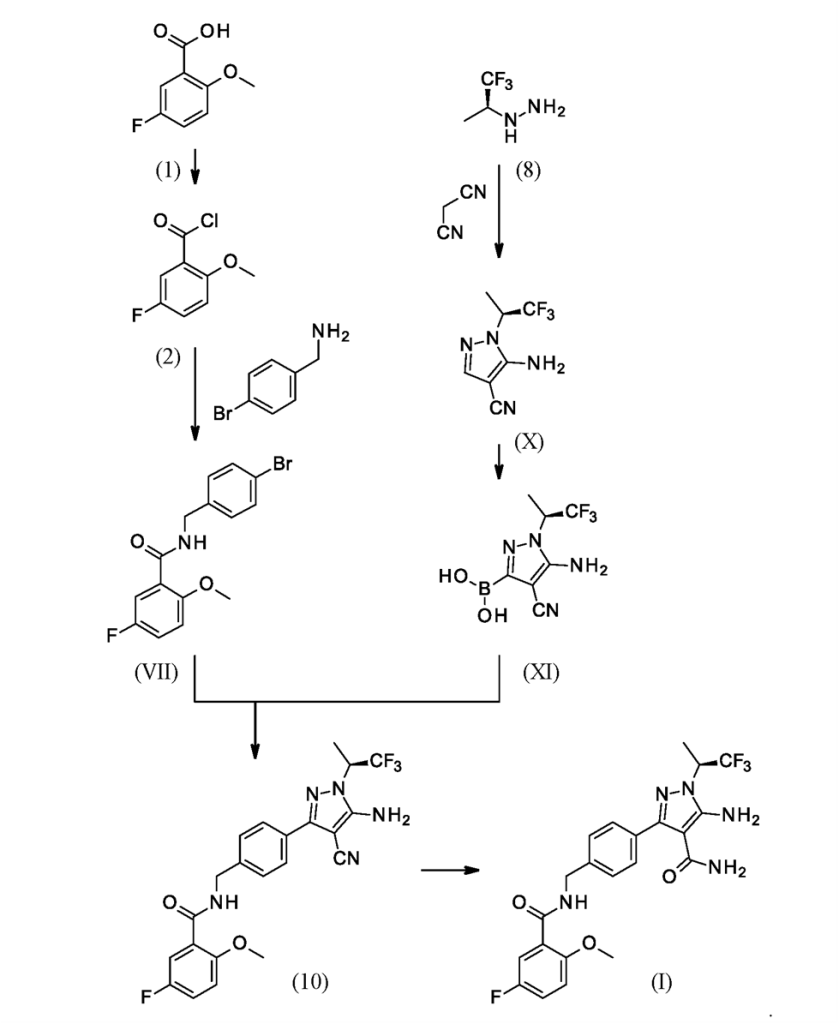
Scheme-5: Ref: WO2022/056100

References: