| Active name: | Leniolisib |
| Brand name: | Joenja |
| Innovator name | PHARMING TECHNOLOGIES BV |
| Approval Date: | March 24, 2023 |
| Therapeutic activity: | phosphoinositide 3-kinase-delta inhibitor that is used to treat activated phosphoinositide 3-kinase delta syndrome |
| Route of adminstration | Tablet, oral |
| Structure | |
| Chemical name | 1-[(3S)-3- [[5,6,7,8-Tetrahydro-6-[6-methoxy-5-(trifluoromethyl)-3-pyridinyl]pyrido[4,3-d]pyrimidin4-yl]amino]-1-pyrrolidinyl]-1-propanone |
| CAS | 1354690-24-6(free base); 1354691-97-6 ( Phosphate salt) |
| Dosage: | 70 mg |
| MDD | 70 mg |
| Molecular weight& molecular formuale | 450.46 & C21H25F3N6O2 |
| OB patents | US 8653092 B (02/19/2032) |
| Polymorhic patent | |
| NCE | 03/24/2028; ODE-430 03/24/2030 |
| Solubility data | Leniolisib phosphate is pH dependent with decreasing solubility observed with increasing pH |
| KEGG | D11159 |
| Drugbank | DB16217 |
| Chemspider | 52083264 |
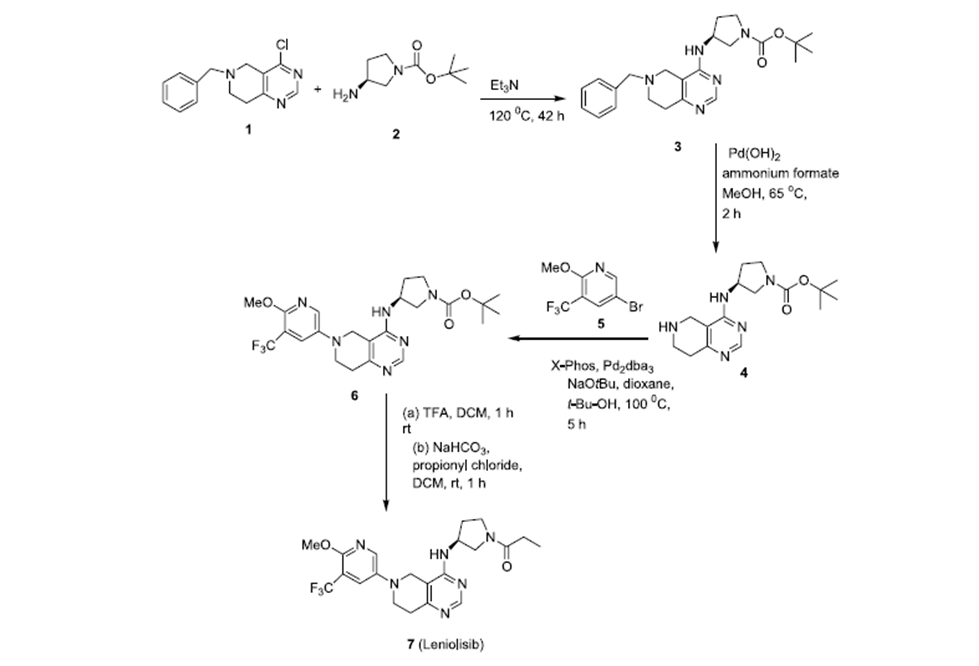
Route of synthesis:
Scheme-1: As per US 8653092B2 reported the synthesis of Leniolisib as scheme-1.
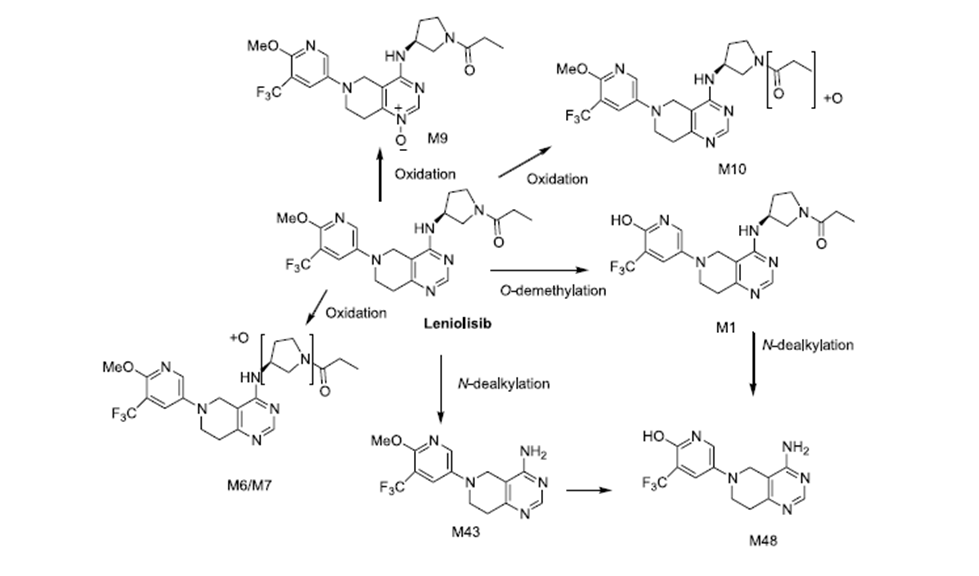
Metabolism of Leniolisib
References:
| Patent No | Patent Expiration | Drug Substance | Drug Product | Submission Date |
| 8653092 | 02/19/2032 | DS | DP | 04/18/2023 |