Product information:
| Active name: | LANDIOLOL HYDROCHLORIDE |
| Brand name: | RAPIBLYK |
| Innovator name | AOP ORPHAN PHARMACEUTICALS AG |
| Approval Date: | NOV 22, 2024 |
| Therapeutic activity: | LANDIOLOL HYDROCHLORIDE is indicated for the treatment of ultra-short-acting β1-selective blocking agent. It inhibits the positive chronotropic effects of catecholamine adrenaline and norepinephrine on the heart. |
| Route of administration | Powder, Intravenous |
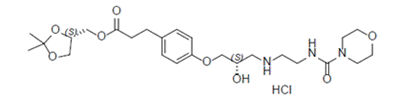
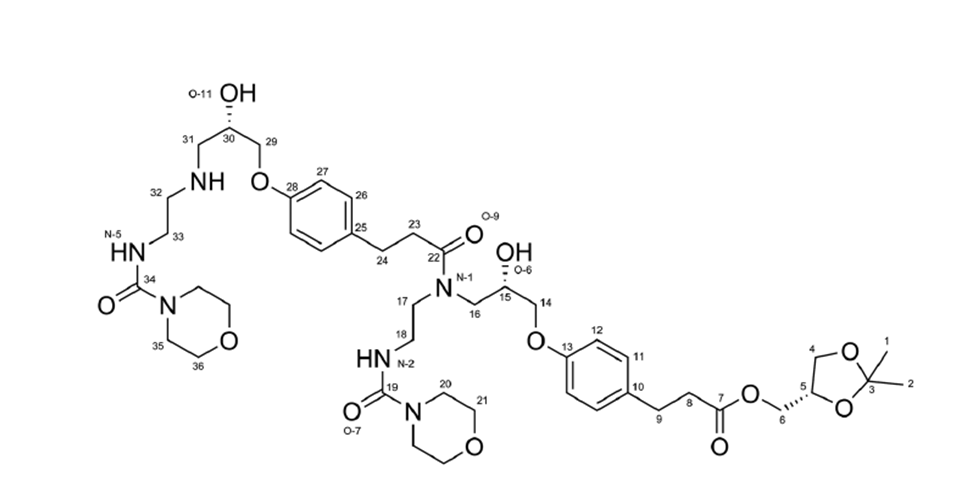
| Structure | |
| Chemical name | [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 3-[4-[(2S)-2-hydroxy-3-[2-(morpholine-4- carbonylamino)ethylamino]propoxy]phenyl]propionate |
| CAS | 133242-30-5 (Free base); 144481-98-1 (Hydro chloride) |
| Dosage | 280 mg base/vial eq (300 mg of hydrochloride) |
| MDD | 225 mg |
| Molecular weight& molecular formuale | 546.06 g/mol & C25H39N3O8.HCl |
| Solubility | Water |
| OB patents | NA. |
| Polymorhic patent | CN 101570531 A |
| NCE | 11/22/2029 |
| Hygroscopicity | Not hygroscopic |
| Drugbank | DB14860 |
| Chemspider | 102855 |
| Pubchem CID | 114905; 164457 |
| Smiles | C(CC(OC[C@H]1OC(C)(C)OC1)=O)C2=CC=C(OC[C@H](CNCCNC(=O)N3CCOCC3)O)C=C2.Cl |
Other information:
Landiolol was approved for medical use in Japan in 2002, in Canada in November 2023 and in the United States in November 2024. It is sold under various brand names including Rapibloc, Raploc, Runrapiq, Landibloc, Onoact, Corbeta, and Rapiblyk.
| IV β-Blocker | max. elimination half-life (min) | cardio-selectivity (β1/β2) | metabilization |
| Landiolol | 4 | 250 | pseudocholinesterases |
| Esmolol | 9 | 30 | ery-esterases |
| Metoprolol | 420 | 3 | cytochrom P2D6 (Leber) |

Synthesis of Landiolol:
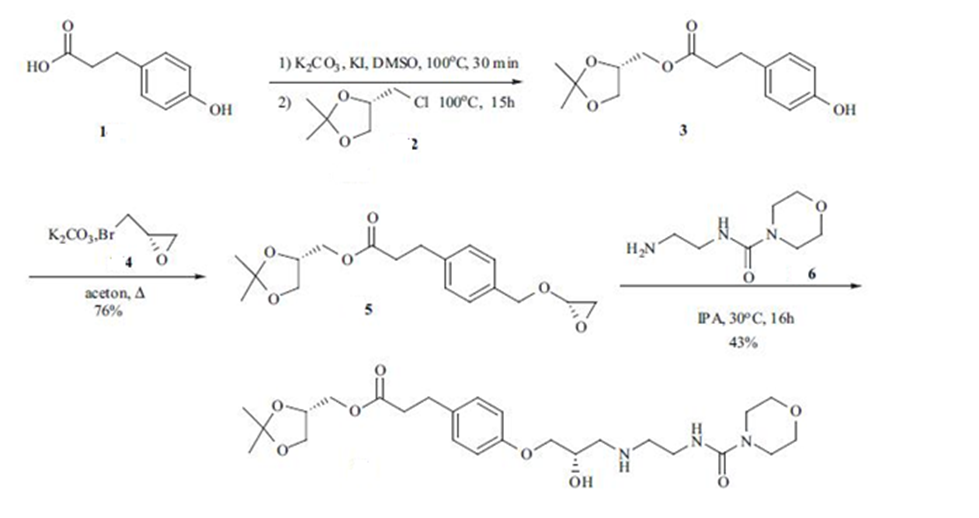
ROS-1: Ref: US 5013734, JP 3302647, CN 100506814, JP 2539734 , Chemical & Pharmaceutical Bulletin 1992, 40 (6) 1462-1469
Synthesis of compound 2 prepared from (S)-epichloro hydrin, which was reported in the earlier(Catalysis Communications, 8(12), 2087-2095; 2007; CN100506814; Journal of Molecular Catalysis A: Chemical, 2005, 236(1-2), 72-76; ). 3-(4-hydroxyphenyl)propionic acid 1 in DMSO solvent to gave the corresponding ester compound 3. Compound 3 couple with chiral epi bromohydrinin 4 in acetone and potassium carbonate to give the compound 5. Compound 5 couple with compound 6 in IPA solvent to achieve the Landiolol.
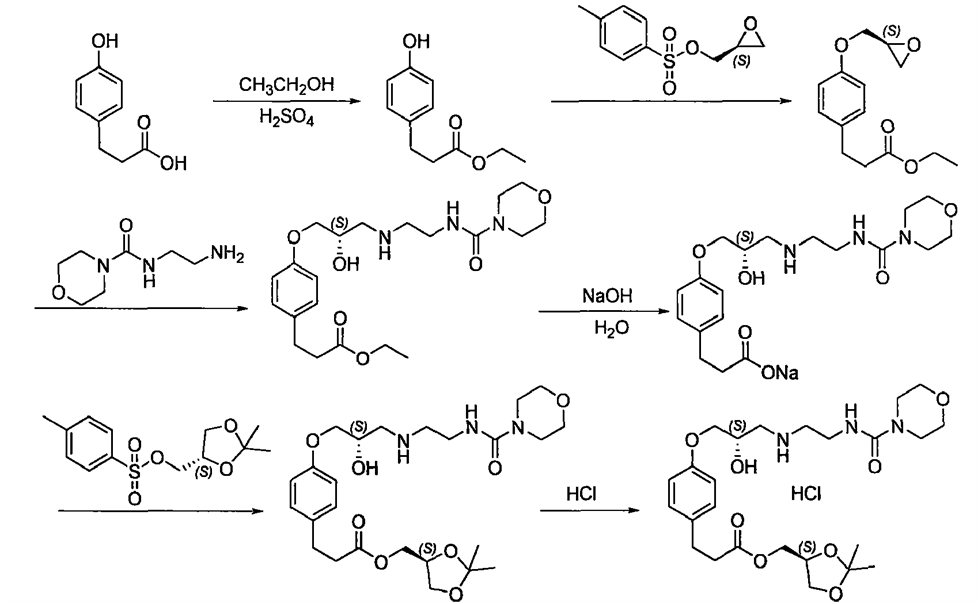
ROS-2: Reference-CN101012217A
Synthesis of Landiolol is prepared from the 3-(4-hydroxyphenyl)propionic acid, which is described in the below scheme .
Impurity structure:
Above impurity synthesis reported by Michal Stujber et al in Magn, Reson. Chem. Let. 2014, 52, 122-127 as described below.