| Active name: | ENSARTINIB HYDROCHLORIDE |
| Brand name: | ENSACOVE |
| Innovator name | XCOVERY HOLDINGS INC |
| Approval Date: | Dec 18, 2024 |
| Therapeutic activity: | ENSACOVE is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC). |
| Route of administration | Capsule, oral |
| Structure | |
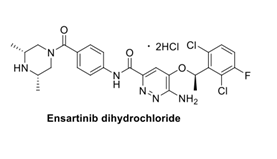
| Chemical name | 6-amino-5-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-N-{4-[(3R,5S)- 3,5-dimethylpiperazine-1-carbonyl]phenyl}pyridazine-3-carboxamide, dihydrochloride |
| CAS | 1370651-20-9 |
| Dosage | 25 mg base eq (28.25 mg of hydrochloride)& 100 mg base eq (113.02 mg of hydrochloride) |
| MDD | 225 mg |
| Molecular weight& molecular formuale | 634.4 g/mol & C26H27Cl2FN6O3.2HCl |
| Solubility | Ethanol, DMSO, DMF |
| OB patents | US8551995B2; US9126947B2;US9296724B2; US10899744B2. |
| Polymorhic patent | US2019/0135792;US2023/0227413A1 |
| NCE | 12/18/2029 |
| Solubility data | Insoluble in water. |
| Hygroscopicity | Not hygroscopic |
| Drugbank | DB14860 |
| Chemspider | 58828042 |
| Pubchem CID | 56960363 |
Patent Data
Orange book Patent Data
Patent Data
| Product No | Patent No | Patent Expiration | Drug Substance | Drug Product | Patent Use Code | Delist Requested | Submission Date |
| 001 | 8551995 | 02/09/2029 | DS | 01/17/2025 | |||
| 001 | 9126947 | 11/29/2031 | DS | 01/17/2025 | |||
| 001 | 9296724 | 06/18/2029 | DS | U-4099 | 01/17/2025 | ||
| 001 | 10899744 | 06/01/2037 | DS | U-4099 | 01/17/2025 |
Synthesis of ENSARTINIB:
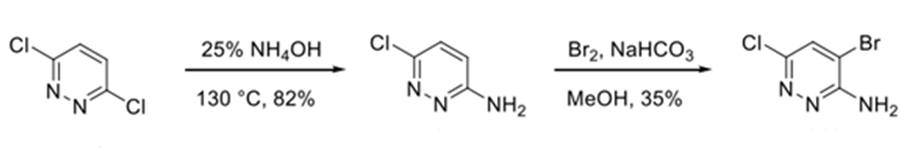
ROS-1: Ref:WO2012/048259
Dichloropyridazine with ammoniumhydroxide furnished amino-pyridazine in 82% yield. The treatment with bromine under basic conditions 4-bromo-6-chloropyridazin-3-amine.
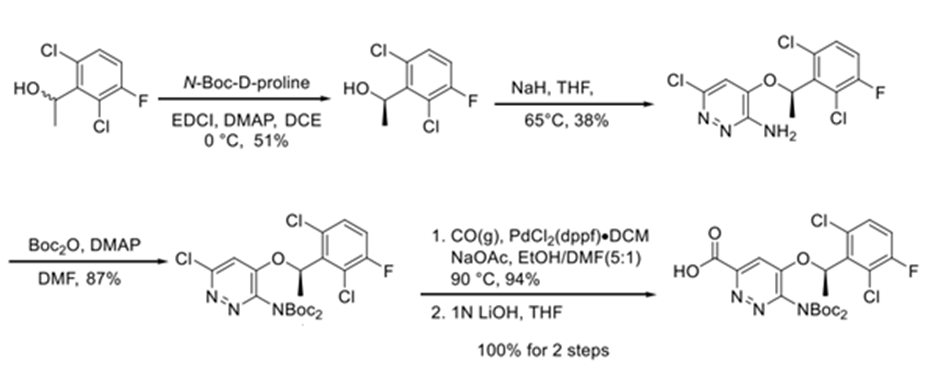
N-Boc-D-proline react with alcohol EDCI-HOBt conditions to deliver ester, then separated isomer 51% yield. Later couple 4-bromo-6-chloropyridazin-3-amine and sodium hydride in THF led the regioselectively (R)-6-chloro-4-(1-(2,6-dichloro-4-fluorophenyl)ethoxy)pyridazin-3-amine. After sequential reactions it converted into Ensartinib dihydrochloride.
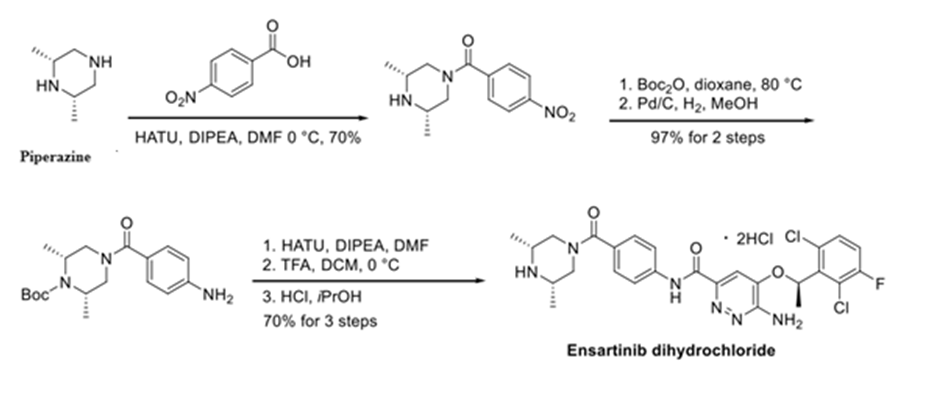
References:
1) WO2009/154769A1
2) CN103298806B
3) US2019/0135792A1