| Active name: | Capivasertib |
| Brand name: | Truqap |
| Innovator name | ASTRAZENECA PHARMACEUTICAL LTD |
| Approval Date: | Nov 16, 2023 |
| Therapeutic activity: | Capivasertib, in combination with fulvestrant, is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer. |
| Route of adminstration | Tablet, oral |
| Structure | |
| Chemical name | 4-amino-N-[(1S)-1-(4- chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinecarboxamide |
| CAS | 11143532-39-1 |
| Dosage | 160 & 200 mg |
| MDD | 320 mg |
| Molecular weight& molecular formuale | 428.92 g/mol & C21H25ClN6O2 |
| OB patents | US 8101623 B2; US9487525 B2; US10059714B2; |
| Polymorhic patent | US9487525 B2; |
| NCE | 11/16/2028 |
| Solubility data | Freely soluble in water when pH below 1.2 and practically insoluble pH above 6.8. |
| Hygroscopicity | Not hygroscopic |
| Pubchem CID | 25227436 |
| Drugbank | DB12218 |
| Chemspider | 28189073 |

| Patent No | Patent Expiration | Drug Substance | Drug Product | Patent Use Code | Delist Requested | Submission Date |
| 8101623 | 03/10/2030 | DS | DP | U-3762 | 12/13/2023 | |
| 8809336 | 10/25/2025 | U-3762 | 12/13/2023 | |||
| 9006430 | 10/25/2025 | DP | 12/13/2023 | |||
| 9487525 | 04/16/2033 | DS | DP | 12/13/2023 | ||
| 10039766 | 04/16/2033 | U-3762 | 12/13/2023 | |||
| 10059714 | 10/10/2028 | DS | DP | 12/13/2023 | ||
| 10654855 | 10/10/2028 | U-3762 | 12/13/2023 | |||
| 11760760 | 10/10/2028 | U-3762 | 12/13/2023 |
Route of synthesis:
Scheme-1:
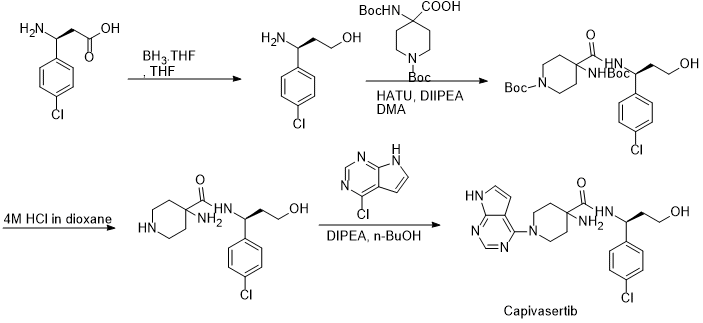
US 8101623B2 reported the synthesis of Capivasertib from 4-chloro phenyl alaninol.
Scheme-2:
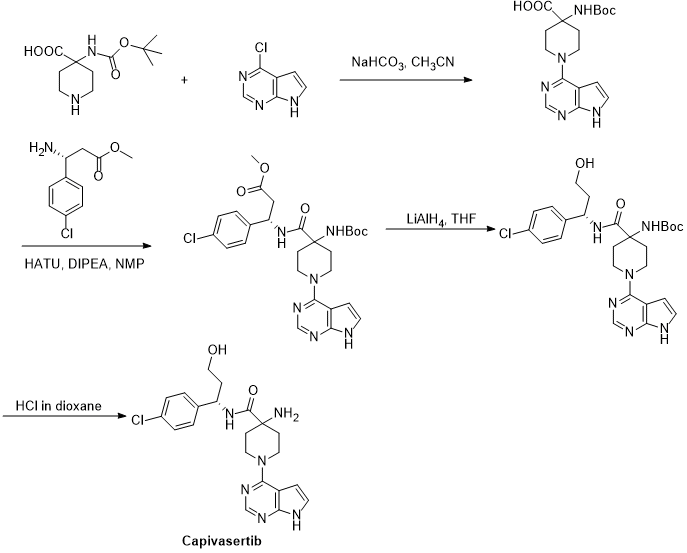
US 8101623B2 reported the synthesis of Capivasertib from 4-chloro-7H-pyrrolo[2,3-d]pyrimidine and 4-(tert-butoxycarbonylamino)piperidine-4-carboxylic acid.
Scheme-3:
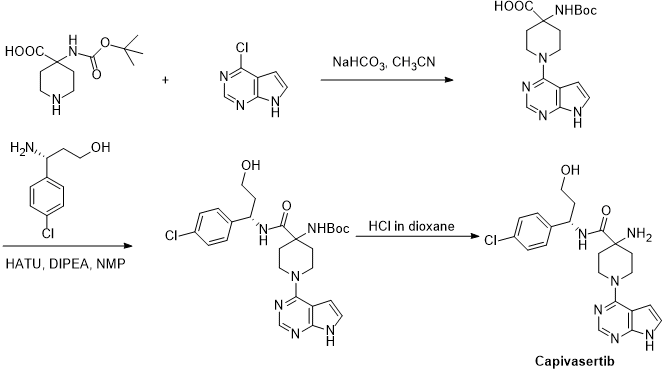
US 8101623B2 reported the synthesis of Capivasertib from 4-chloro-7H-pyrrolo[2,3-d]pyrimidine and 4-(tert-butoxycarbonylamino)piperidine-4-carboxylic acid.
References:
ournal of Medicinal Chemistry
Cite this: J. Med. Chem. 2013, 56, 5, 2059–2073
https://doi.org/10.1021/jm301762v